is saline solution a homogeneous mixture
A solution is a homogeneous mixture of two. View all Which is an example of a homogeneous mixture.
What are five examples of homogeneous mixtures.

. Carbonated water vodka and saline are all examples of homogeneous solutions. Which is a homogeneous mixture. Homogeneous mixtures ESAY A solution of salt dissolved in water is an example of a homogeneous mixture.
Normal saline solution synonyms normal saline solution pronunciation normal saline solution translation English dictionary definition of normal saline solution. Solution in chemistry homogeneous mixture mixture in chemistry a physical combination of two or more pure substances ie elements or compounds. Examples of heterogeneous mixtures include sand oil and water and chicken noodle soup.
A mixture is said to be homogenous if it has a uniform composition throughout and there are no visible boundaries of operation between constituents. Homogeneous mixtures are sources of. Sought a solution to falling enrollments.
Examples of homogeneous mixtures include air saline solution most alloys and bitumen. Define normal saline solution. Saline solution is sodium chloride at 085 to 09 added to and dissolved in 100 mL of purified water.
A solution is a mixture of two or more substances in a single phase. All solutions would be considered homogeneous because the dissolved material is present in the same amount throughout the solution. Sand oil and water and chicken noodle soup are examples of heterogeneous mixtures.
Solid Homogeneous Mixture Examples. Homogeneous mixtures are also known as solutionsWhen you think of a solution you probably think of a liquid. Through combining two or more substances a mixture is produced.
When the salt dissolves it spreads evenly through the water so that all parts of the solution are the same and you can no longer see the salt as being separate from the water. Through combining two or more substances a mixture is produced. Homogeneous mixtures are sources of water saline solution some alloys and bitumen.
- A homogeneous mixture that contains large particles - A heterogeneous mixture of large particles in a liquid. Homogeneous mixtures ESAY A solution of salt dissolved in water is an example of a homogeneous mixture. A homogeneous solution is a mixture of two or more components that have a uniform appearance and composition.
The amount of salt in the salt water can vary from one sample to another. A heterogeneous mixture of large particles in a liquid. Physiological saline solution E.
In chemistry a solution is a homogeneous mixture containing two or more substances in which a solute is the substance that is dissolved in a solvent. Examples include common salt dissolved in water saline water sugar dissolved in water iodine dissolved in CCl4 Benzene in toluene and Methyl alcohol in water. Liquid examples include pure water white vinegar sugar water corn oil and blood.
Examples of heterogeneous mixtures include sand oil and water and chicken noodle soup. What are some examples of homogeneous mixtures. SOLUTIONS are homogeneous mixtures.
In chemistry a solution is a homogeneous mixture containing two or more substances in which a solute is the substance that is dissolved in a solvent. Solution in chemistry homogeneous mixture mixture in chemistry a physical combination of two or more pure substances ie elements or compounds. Sometimes homogeneous mixtures are called solutions.
At least two substances must be mixed in order to have a solution. How many grams of sodium chloride are contained in 500 mL of this solution. Examples of homogeneous mixtures include air saline solution most alloys and bitumen.
A method or process of dealing with a problem. A homogeneous solution tends to be identical no matter how you sample it. Homogeneous solutions should not be confused with heterogeneous suspensions in which the components are made up of larger and less-uniform particles.
A homogeneous solution tends to be identical no matter how you sample it. Examples of homogeneous mixtures include air saline solution most alloys and bitumen. The substance in the smallest amount and the one that dissolves or disperses is called the SOLUTE.
One characteristic of mixtures is that they can be separated into their components. A mixture is distinguished from a compound which is formed by the chemical combination of two or more pure substances in a fixed definite proportion. Homogeneous mixtures have a uniform composition due to their evenly distributed particles.
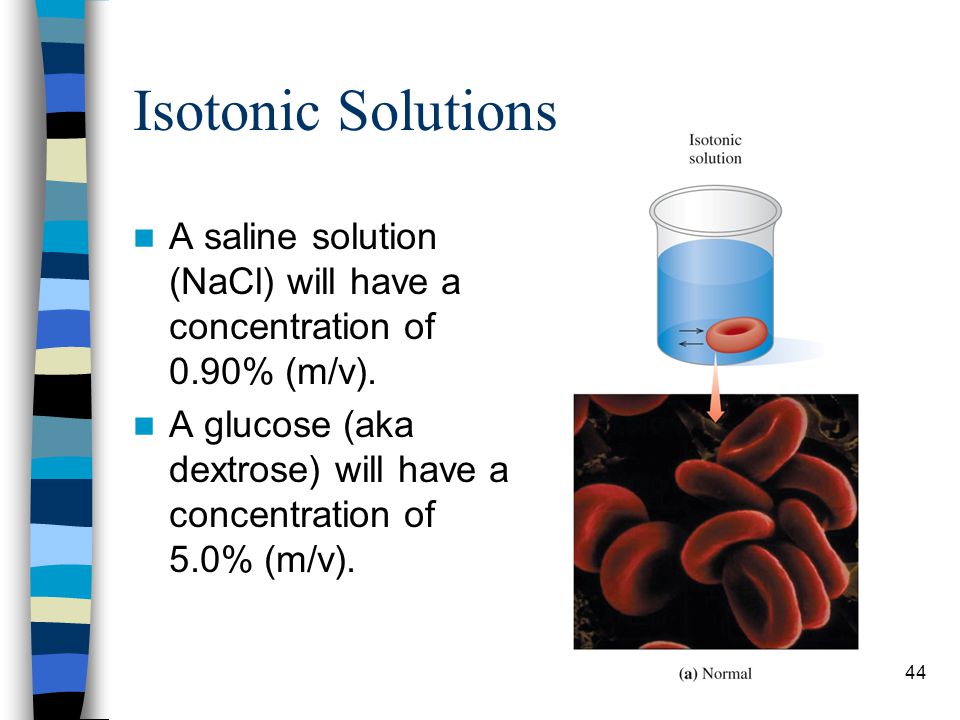
A saline solution contains 092 wv sodium chloride in water. Examples of homogeneous mixtures include air saline solution most alloys and bitumen. When the salt dissolves it spreads evenly through the water so that all parts of the solution are the same and you can no longer see the salt as being separate from the water.
However many solids are also considered homogenous mixturesThere is a wide variety of solid homogeneous mixtures from naturally occurring materials like stone to synthetic plastics. Learn about the definition of. A homogeneous mixture is a solid liquid or gaseous mixture.
Examples of heterogeneous mixtures include sand oil and water and chicken noodle soup. Salt water solution is a homogeneous mixture for example but salt mixed with sand is a heterogeneous mixture. Why is saline solution a homogeneous mixture.
What are homogeneous and heterogeneous mixture give examples. A homogeneous mixture appears uniform regardless of where you sample it. What is homogeneous solution give example.
Saline solution is sodium chloride at 085 to 09 added to and dissolved in 100 mL of purified water. A mixture is distinguished from a compound which is formed by the chemical combination of two or more pure substances in a fixed definite proportion.
Saline Solution For Piercings How To Discuss

Solved 5 Label Each Of The Following As A Homogeneous Chegg Com

Solved Q Classify The Following As Homogeneous Chegg Com

12 Best Examples Of Homogeneous Mixtures Rankred

Saline Solution For Piercings How To Discuss

Biochemistry Practicals Paramedics World
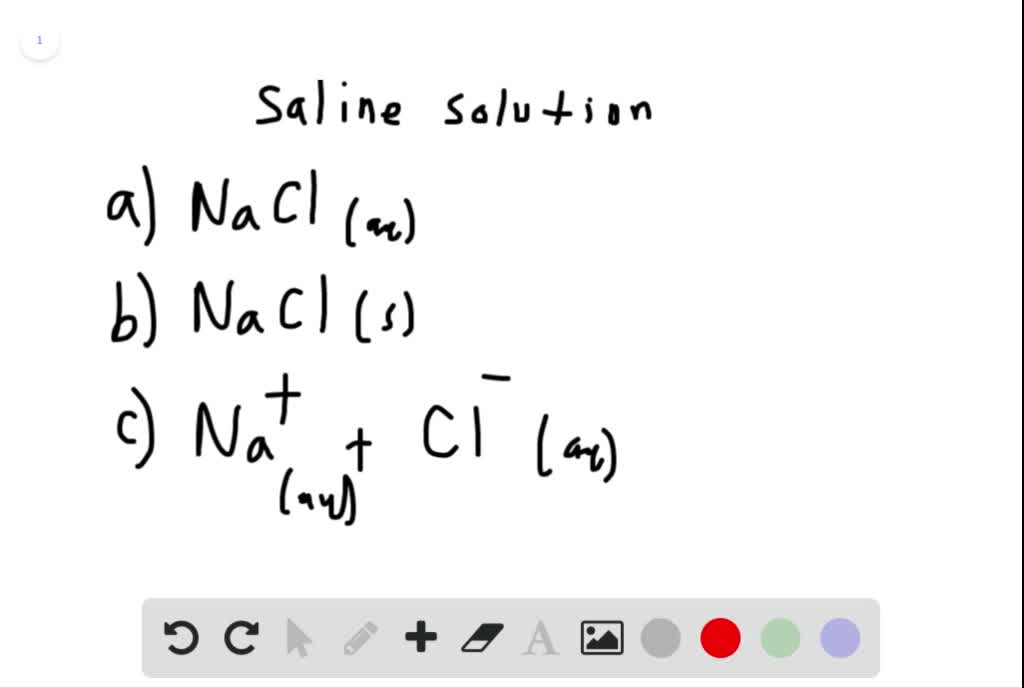
Solved The Saline Solution Used To Soak Contact Lenses Is Primarily Nacl Dissolved In Water Which Of The Following Ways To Represent The Solution Is Not Correct Begin Array L Text A Operatorname Nacl A Q

Pin By Hannah Saxton On Intriguing Thoughts Thoughts Spirituality Instagram

Venn Diagram Create A Venn Diagram For Solution Colloid And Suspension You Will Need To Include Particle Size Type Of Mixture Homogeneous Or Heterogeneous Ppt Download
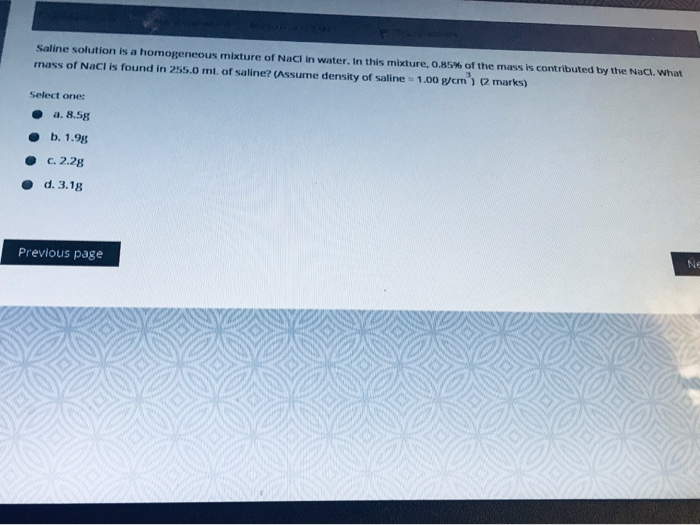
Solved Saline Solution Is A Homogeneous Mixture Of Nacl In Chegg Com

Equate Contact Lens Saline Solution For Sensitive Eyes T Sensitive Eyes Saline Solution Contact Solution

Chapter 7 Solutions Section 1 Solutions Vs Mixtures Sodium Chloride Liquid Solution Air Is A Gaseous Solution Ppt Download

Properties Of Solutions Part B Liquid Solutions Clear
Chapter 7 Solutions A Solution Is A Homogeneous Mixture That Consists Of The Solute And The Solvent Ppt Video Online Download

Consider The Following Substance I Saline Clutch Prep

Pin By Linda Mann On Crafts Liquid Food Coloring Contact Lens Solution School Glue

Chapter 7 Solutions A Solution Is A Homogeneous Mixture That Consists Of The Solute And The Solvent Ppt Video Online Download



0 Response to "is saline solution a homogeneous mixture"
Post a Comment